Multimodal analysis of novel mouse models associated with Glutamate transporter mutations
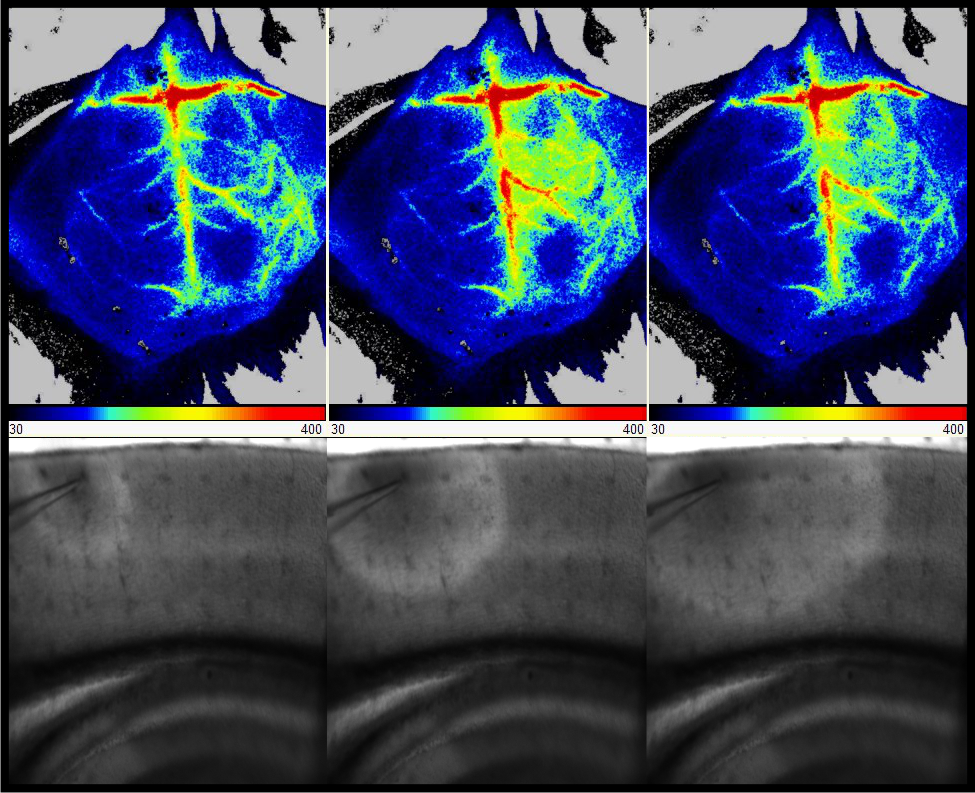
Hemiplegic migraine (HM) is a severe monogenic subtype of migraine with aura and hemiparesis. Three different causative genes have been established (CACNA1A, ATP1A2 and SCN1A). Using transgenic knock-in animals, we and others could show that mutations in these genes increase the susceptibility to cortical spreading depolarization (CSD). CSD, a wave of synchronous neuronal depolarizations slowly propagating across the cortex, is the correlate of migraine aura.
Genetic variations in SCL1A3, encoding the astrocytic glutamate transporter EAAT1, has been implicated in several neurological disorders. Specifically, we detected the variant T387P in a HM patient and demonstrated complete EAAT1 loss-of-function in cellular analyses. Here, we aim at gaining a comprehensive understanding how altered glutamate homeostasis caused by EAAT1 dysfunction contributes to the pathophysiology of this potential novel HM subtype. As a model for T387P associated EAAT1 loss-of-function we will use Slc1a3+/- mice; for comparison, we will use a knock-in mouse for a different SCL1A3 mutation (P290R), associated with another, more complex clinical phenotype. In both mice, we will perform electrophysiological recordings in acute slices, perform video EEG recording and analyze the susceptibility to experimentally induced CSD in vivo. Results will be subjected to a head-to-head comparison with data obtained in a Scn1a knock-in mouse model for HM which is currently under analysis in our group. Finally, our project will provide a platform for in vivo CSD analysis also to other members of the consortium, thereby allowing the evaluation of novel therapeutic targets.